Iodometric Titration Experiment Using Sodium Thiosulphate . We generate a measured excess of iodine in the sample. The amount of iodine liberated in the reaction. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. two methods are commonly used to determine do concentration: the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. Numerous methods are based upon the reducing properties of iodide ion: (1) the iodometric method which is a titration. At the moment that all of the elemental iodine. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine.
from www.numerade.com
two methods are commonly used to determine do concentration: a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. The amount of iodine liberated in the reaction. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. We generate a measured excess of iodine in the sample. the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. At the moment that all of the elemental iodine. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. Numerous methods are based upon the reducing properties of iodide ion: (1) the iodometric method which is a titration.
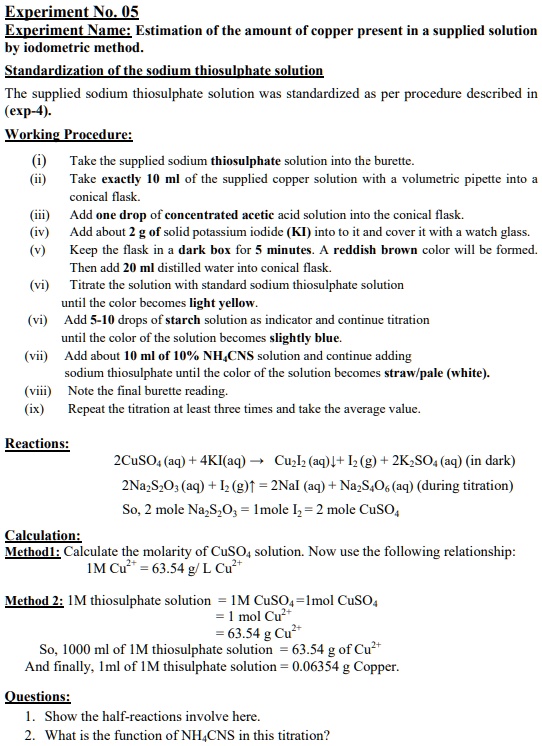
Experiment No.05 Experiment Name Estimation of the amount of copper
Iodometric Titration Experiment Using Sodium Thiosulphate a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. The amount of iodine liberated in the reaction. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. two methods are commonly used to determine do concentration: We generate a measured excess of iodine in the sample. (1) the iodometric method which is a titration. Numerous methods are based upon the reducing properties of iodide ion: At the moment that all of the elemental iodine. the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Iodometric Titration Experiment Using Sodium Thiosulphate the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. Numerous methods are based upon the reducing properties of iodide ion: We generate a measured excess of iodine in the sample. two methods are commonly used to determine do concentration: thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry.. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.academia.edu
(DOC) Experiment no. 1 Iodometric titration of potassium dichromate Iodometric Titration Experiment Using Sodium Thiosulphate We generate a measured excess of iodine in the sample. (1) the iodometric method which is a titration. the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. Numerous methods are based upon the reducing properties of iodide ion: At the moment that all of the elemental iodine. The amount of iodine liberated in the reaction. a. Iodometric Titration Experiment Using Sodium Thiosulphate.
From hyprowira.com
Iodometric Titration Functions and How It Works Iodometric Titration Experiment Using Sodium Thiosulphate the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. We generate a measured excess of iodine in the sample. At the moment. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.youtube.com
Iodimetric titration standardization of thiosulfate YouTube Iodometric Titration Experiment Using Sodium Thiosulphate The amount of iodine liberated in the reaction. We generate a measured excess of iodine in the sample. two methods are commonly used to determine do concentration: the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. (1) the iodometric method which is a titration. a precise and stable reducing agent, sodium thiosulfate (na 2 s. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.youtube.com
Sodium Thiosulphate Solution preparation and Standardization Iodometric Titration Experiment Using Sodium Thiosulphate the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. two methods are commonly used to determine do concentration: The amount of iodine liberated in the reaction. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. We generate a measured excess of iodine in the sample. At the moment. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.numerade.com
Experiment No.05 Experiment Name Estimation of the amount of copper Iodometric Titration Experiment Using Sodium Thiosulphate two methods are commonly used to determine do concentration: We generate a measured excess of iodine in the sample. (1) the iodometric method which is a titration. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. thanks to its relatively low, ph independent redox potential,. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.youtube.com
Preparation & standardization of Sodium Thiosulphate/Iodometric Iodometric Titration Experiment Using Sodium Thiosulphate Numerous methods are based upon the reducing properties of iodide ion: the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. The amount of iodine liberated in the reaction. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. We generate a measured excess of iodine in the sample. . Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.youtube.com
How to do Redox Titration in Hindi Iodometric Titration of Copper Iodometric Titration Experiment Using Sodium Thiosulphate We generate a measured excess of iodine in the sample. the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. At the moment that all of the elemental iodine. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. Numerous methods are based upon the reducing properties of iodide ion: The. Iodometric Titration Experiment Using Sodium Thiosulphate.
From mavink.com
Sodium Thiosulfate Titration Iodometric Titration Experiment Using Sodium Thiosulphate two methods are commonly used to determine do concentration: We generate a measured excess of iodine in the sample. The amount of iodine liberated in the reaction. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.scribd.com
Standardization of Sodium Thiosulfate Solution and Determination of Iodometric Titration Experiment Using Sodium Thiosulphate Numerous methods are based upon the reducing properties of iodide ion: another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. (1) the iodometric method which is a titration. a precise and stable reducing agent, sodium thiosulfate (na 2. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.youtube.com
N/10 Sodium Thiosulfate Solution Preparation and Standardization with Iodometric Titration Experiment Using Sodium Thiosulphate the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. We generate a measured excess of iodine in the sample. The amount of. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.youtube.com
Iodometric Estimation of Copper using Sodium thiosulphate YouTube Iodometric Titration Experiment Using Sodium Thiosulphate the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. two methods are commonly used to determine do concentration: The amount of iodine liberated in the reaction. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. At the moment that all of the elemental iodine. We generate a. Iodometric Titration Experiment Using Sodium Thiosulphate.
From www.youtube.com
11 Standardization of Sodium Thiosulphate using Iodometric Titration Iodometric Titration Experiment Using Sodium Thiosulphate two methods are commonly used to determine do concentration: The amount of iodine liberated in the reaction. We generate a measured excess of iodine in the sample. the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with. Iodometric Titration Experiment Using Sodium Thiosulphate.
From dxojsbiep.blob.core.windows.net
Iodometric Titration Of Iodine And Sodium Thiosulphate at Stacie King blog Iodometric Titration Experiment Using Sodium Thiosulphate a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. We generate a measured excess of iodine in the sample. another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. At the moment that all of the elemental iodine. The. Iodometric Titration Experiment Using Sodium Thiosulphate.
From shopee.ph
Sodium Thiosulfate Standard Solution Iodometric Titration Analysis Iodometric Titration Experiment Using Sodium Thiosulphate The amount of iodine liberated in the reaction. We generate a measured excess of iodine in the sample. At the moment that all of the elemental iodine. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. Numerous methods are based upon the reducing properties of iodide ion: a precise and stable. Iodometric Titration Experiment Using Sodium Thiosulphate.
From dxojsbiep.blob.core.windows.net
Iodometric Titration Of Iodine And Sodium Thiosulphate at Stacie King blog Iodometric Titration Experiment Using Sodium Thiosulphate Numerous methods are based upon the reducing properties of iodide ion: The amount of iodine liberated in the reaction. the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. two methods are commonly used to determine do concentration: thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. We generate. Iodometric Titration Experiment Using Sodium Thiosulphate.
From mymumbaipost.in
Explore 10 Key Difference between Iodometry and Iodimetry Mymumbaipost Iodometric Titration Experiment Using Sodium Thiosulphate another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. At the moment that all of the elemental iodine. The amount of iodine liberated in the reaction. (1) the iodometric method which is a titration. the sodium thiosulfate reacts with elemental iodine to produce sodium iodide. thanks to its relatively. Iodometric Titration Experiment Using Sodium Thiosulphate.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Iodometric Titration Experiment Using Sodium Thiosulphate another application of iodometric titration involves the estimation of cu(ii) (copper(ii) oxide) using a sodium thiosulfate solution. (1) the iodometric method which is a titration. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. We generate a measured excess of iodine in the sample. The amount. Iodometric Titration Experiment Using Sodium Thiosulphate.